Our addiction to our cell phones, laptops, the growth of electric vehicles and other personal electronic devices drives an increased market need for more batteries.
As our use cases for batteries grow, so too does our need for recycling these critical and rare elements.
There’s a great quote about battery recycling: “The largest lithium and cobalt reserves in the western hemisphere [are] locked away in America’s junk drawers”.
Our iPhone and other consumer electronic batteries have used LCO as the cathode material since commercialization in the 1980s. How could we make sure that these iPhone batteries are contributing to a circular American supply chain? What if once cobalt made it to the US, it never left?
There are a few companies working on battery recycling to reduce the environmental impact of manufacturing batteries, while also lowering demand for rare materials.
There are three main types of battery recycling: pyrometallurgical, hydrometallurgical, and direct recycling.
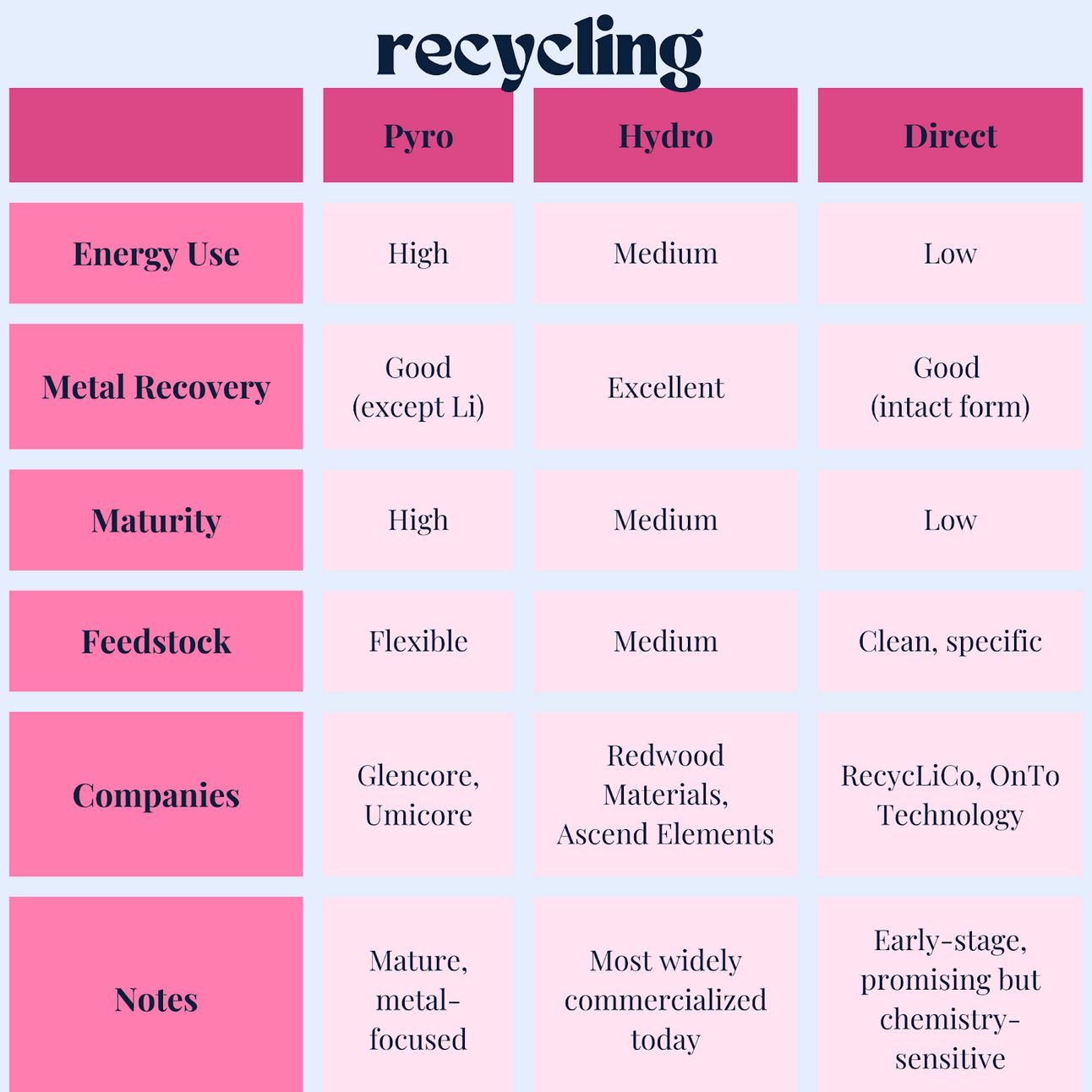
Pyrometallurgical Recycling
What it is:
High-temperature processing (smelting) to extract valuable metals
How it works:
Batteries are shredded or incinerated
Organic materials and plastics burn off
Metals melt and separate based on density
What you get:
Recover metals like cobalt, nickel, and copper as alloys or oxides
Lithium and aluminum are often lost or go to slag (smelting byproduct)
Pros:
Mature and industrially scalable
Handles mixed chemistries easily
Cons:
Energy-intensive (high heat)
Poor lithium recovery
Destroys cathode structure, so no reuse of active materials
Hydrometallurgical Recycling
What it is:
Uses acid or base solutions to dissolve and recover metals
How it works:
Batteries are disassembled/shredded
Leaching agents dissolve the metals
Metals are selectively precipitated or extracted
What you get:
High purity recovery of cobalt, nickel, lithium, manganese
Pros:
Lower energy use than pyro
Good lithium recovery
Scalable and adaptable to different chemistries
Cons:
Generates chemical waste
Still destroys cathode structure
More complex separation process
Direct Recycling
What it is:
Tries to preserve and rejuvenate the cathode material directly
How it works:
Batteries are carefully disassembled
Cathode powder is harvested
Physical and chemical methods restore structure and composition
What you get:
Reconditioned cathode ready for reuse
Pros:
Lowest energy use
Maintains material structure, ideal for reuse
Potentially cheapest and most sustainable long term
Cons:
Not yet mature at scale
Chemistry-specific, harder with mixed-streams
Requires clean, well-sorted feedstock
Conventional battery recycling methods—such as pyrometallurgy and hydrometallurgy—break down batteries to recover individual metals. These metals are then reprocessed into battery-grade precursors, effectively serving as a form of "urban mining." In contrast, direct recycling aims to recover and restore battery components—particularly the cathode—while maintaining their original crystal structures. This approach avoids the energy- and cost-intensive steps of breaking down and re-synthesizing materials. [1]
Pyrometallurgical and hydrometallurgical methods for recycling spent lithium-ion batteries are moving toward commercial use.
Companies using pyro: Li-Cycle (initially), Umicore, Glencore.
Companies using hydro: Li-Cycle, Redwood Materials, Ascend Elements
Companies direct recycling: RecycLiCo (American Manganese), Battery Resourcers (merged with Ascend), OnTo Technology, Princeton NuEnergy
According to the EverBatt model, direct recycling offers significant economic and environmental advantages. Producing 1 kg of NMC111 cathode via direct recycling results in a 44% cost reduction compared to using virgin materials. It also cuts energy consumption by 14% compared to hydrometallurgical recycling and 25% compared to pyrometallurgical methods.[2]
Lab-scale demonstrations of direct recycling have shown promising results across various chemistries, including LFP[3], LCO[4], NMC[5,6–9], and graphite[10]. However, one of the major hurdles to commercialization is the manual separation of battery components, which is labor-intensive and inefficient at scale [1,2].
Unlike pyrometallurgical and hydrometallurgical processes—which typically grind and mix battery cells into a composite “black mass”—direct recycling requires careful disassembly. Individual cell components, such as the cathode and anode, must be separated cleanly. For example, recovering cathode material often involves detaching active mass from the aluminum current collector, commonly using solvents like NMP to dissolve the PVDF binder.
A shift toward a standardized cell design could significantly enhance the commercial viability of direct recycling, enabling automation and reducing processing complexity. [2,7-10]
Some Resources We Love :
This paper on NMR analysis of recycled battery materials has great background on NMC recycling and also covers upcycling to higher Ni content NMC.
Sources
1. Toro, L. et al. A Systematic Review of Battery Recycling Technologies: Advances, Challenges, and Future Prospects. Energies 16, 6571 (2023).
2. Ma, X. et al. The evolution of lithium-ion battery recycling. Nat. Rev. Clean Technol. 1, 75–94 (2025).
3. Xu, P. et al. Efficient Direct Recycling of Lithium-Ion Battery Cathodes by Targeted Healing. Joule 4, 2609–2626 (2020).
4. Sloop, S. et al. A direct recycling case study from a lithium-ion battery recall. Sustain. Mater. Technol. 25, e00152 (2020).
5. Gupta, V. et al. Scalable Direct Recycling of Cathode Black Mass from Spent Lithium-Ion Batteries. Adv. Energy Mater. 13, 2203093 (2023).
6. Montoya, A. T. et al. Direct Recycling of Lithium-Ion Battery Cathodes: A Multi-Stage Annealing Process to Recover the Pristine Structure and Performance. ACS Sustain. Chem. Eng. 10, 13319–13324(2022).
7. Shi, Y., Zhang, M., Meng, Y. S. & Chen, Z. Ambient-Pressure Relithiation of Degraded LiNi0.5Co0.2Mn0.3O2 (0 < x < 1) via Eutectic Solutions for Direct Regeneration of Lithium-Ion Battery Cathodes. Adv. Energy Mater. 9, 1900454 (2019).
8. Shi, Y., Chen, G., Liu, F., Yue, X. & Chen, Z. Resolving the Compositional and Structural Defects of Degraded LiNixCoyMnzO2 Particles to Directly Regenerate High-Performance Lithium-Ion Battery Cathodes. ACS Energy Lett. 3, 1683–1692 (2018).
9. Gao, H. et al. Upcycling of Spent LiNi0.33Co0.33Mn0.33O2 to Single-Crystal Ni-Rich Cathodes Using Lean Precursors. ACS Energy Lett. 8, 4136–4144 (2023).
10. Markey, B. et al. Effective Upcycling of Graphite Anode: Healing and Doping Enabled Direct Regeneration. J. Electrochem. Soc. 167, 160511 (2020).